Energy Models
To support decisions about our energy systems, we must make quantitative estimates in systems that are too complex to gather complete information.
Units
One joule is the energy delivered by one watt of power running for one second. This unit is often used by scientists.
One kilowatt-hour is the energy delivered by one kilowatt of power running for one hour. This unit is used by electric utilities on consumer bills.
Energy Metrics and Analysis Tools
There are several types of energy quantities that come up frequently. How do you decide which of these quantities is relevant to your estimation and decision?
Here is a list of types of quantities and their uses
- Unit Conversions
- convert a quantity from one unit to another, keeping the dimension the same
- Density
- converts volumes to masses
- Energy Density
- calculates the volume or mass required to generate an amount of energy
- A gravimetric energy density relates energy and mass
- A volumetric energy density relates energy and volume
- Efficiency
- determine how much of one type of energy can be converted to another
- Carbon Intensity
- calculates the carbon released from a given amount of energy
- Be aware that the number may be for the mass of carbon dioxide or the mass of carbon.
- Power and Energy Density per Unit Area
- Often used for renewable energy
- Power available per area of land or rooftop
- Energy available per area of land or rooftop (in a given time period)
Unit Conversions
Usually when we create an estimation, the dimensions are fixed but we have a choice of what units we use.
It may be simplest to perform the calculation using units that are correct, but not the best for communicating with an audience.
If this is the case we can use a unit conversion to go from the correct but awkward unit to a better unit for communication.
Example
Suppose someone says it is 640,000 inches to drive from SSU to Petaluma. This is correct, but you might not find it helpful. If we know that there are 5,280 feet in a mile and 12 inches in a foot, we can provide a more familiar unit.
640,000\ \textrm{inches} \cdot \frac{1\ \textrm{foot}}{12\ \textrm{inches}} \cdot \frac{1\ \textrm{mile}}{5280\ \textrm{feet}} = 10.1\ \textrm{miles}
Units and Dimensions
Note that a unit conversion has different units on the top and bottom and the same dimensions on top and bottom.
This means multiplying a quantity by a unit conversion changes the units but doesn’t change the dimension.
Common Unit Conversions
1609 meters = 1 mile (length)
1055 Joules = 1 BTU (energy)
3.6 \times 10^6 Joules = 1 kWh
Standard Multiplier Prefixes and Scientific Notation
Often for energy quantities, we use the metric prefixes to express scientific notation.
Rather than say 3.2 \cdot 10^{9} Joules, will say 3.2 GJ, using the G for giga or billion.
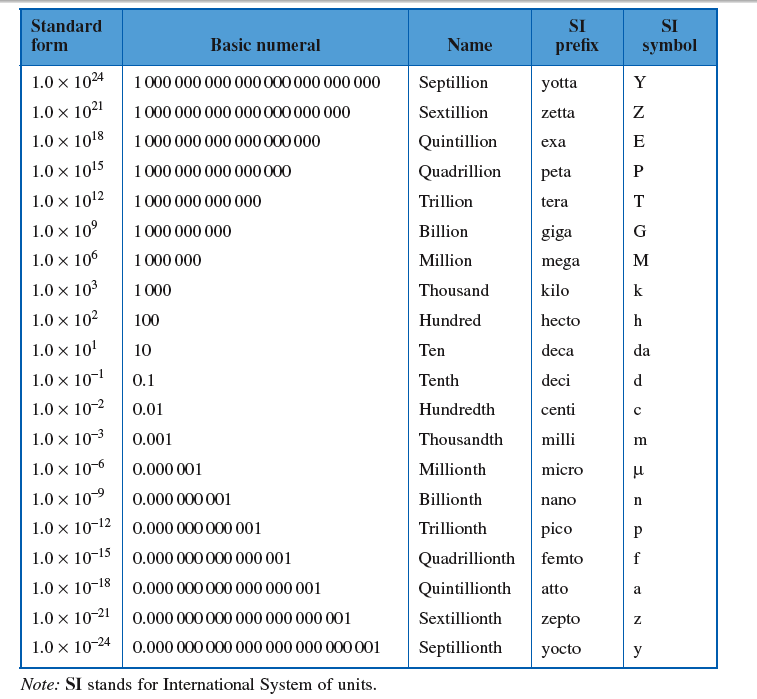
In the same way as we have before, we can create conversion factors using the table above.
1\ \textrm{GJ} = 1000\ \textrm{MJ}
\frac{1\ \textrm{GJ}}{1\ \textrm{GJ}}= \frac{1000\ \textrm{MJ}}{1\ \textrm{GJ}}
The fraction on the right can be used the same way a unit conversion fraction can.
10\ \textrm{GJ} \cdot \frac{1000\ \textrm{MJ}}{1\ \textrm{GJ}} = 10,000\ \textrm{MJ}
| Scientific Notation | English | SI prefix | Symbol | Mnemonic |
|---|---|---|---|---|
| 10^{24} | Septillion | yotta | Y | yodeling |
| 10^{21} | Sextillion | zetta | Z | zebras |
| 10^{18} | Quintillion | exa | E | expect |
| 10^{15} | Quadrillion | peta | P | patient |
| 10^{12} | Trillion | tera | T | teachers |
| 10^{9} | Billion | giga | G | giving |
| 10^{6} | Million | mega | M | metric |
| 10^{3} | Thousand | kilo | k | knowledge |
Unit Conversion Example
How many joules in 100 BTU?
From tables or the internet we find 1 BTU = 1055 Joules.
100 BTU \cdot \frac{1055 J}{1 BTU} \cdot \frac{1kJ}{1000J} = 106 kJ
Density
A density converts a mass to a volume or a volume to a mass.
| Material | Density(g/cubic centimeter) |
|---|---|
| Crude Oil | ~0.9 |
| Water | 1.0 |
| Air | 0.0012 |
| Gasoline | 0.740 |
Energy Density
Gravimetric Energy Density
This is the quantity of energy is released by the conversion (often combustion) of a given mass of the material. Here is a table of the gravimetric (mass) energy densities for a few popular energy storage sources.
| Material | Energy Density (MJ/kg) |
|---|---|
| Gasoline | 45 |
| Crude oil | 42–44 |
| Natural gas | 33–37 |
| Coal | 12–31 |
| Wood | 14–16 |
| Lithium Battery | 0.5 |
Units and Dimensions
An energy density has different dimensions and different units on the top and bottom. The dimensions of a mass energy density are energy over mass.
This means multiplying by an energy density changes the dimension of a quantity unlike a unit conversion.
Volumetric Energy Density
This is the quantity of energy that is released by a given volume of the material.
Carbon Intensity
Carbon intensity is defined as the carbon released divided by the amount of services provided. This could be mass of CO2 per mile driven, or mass of CO2 per unit of electricity generated.
These are averages for the carbon intensity of electricity for some power plants.
| Fuel Source | Carbon Intensity |
|---|---|
| Coal | 1001 gram CO2/kWh |
| Natural Gas | 469 gram CO2/kWh |
| Biomass | 230 gram CO2/kWh |
| Solar PV | 46 gram CO2/kWh |
| Geothermal | 45 gram CO2/kWh |
| Nuclear | 16 gram CO2/kWh |
| Wind | 12 gram CO2/kWh |
| Hydroelectric | 4 gram CO2/kWh |
Median intensity from IPCC 2011 Annex II.
Energy to Power
If I convert 1000 joules of energy in 10 seconds, how many watts of power is this?
Power to Energy
If we have a 50 watt laptop running for a short 100 second video we find the energy used by
E = P \cdot t
E = 50\ \textrm{watt} \cdot 100\ \textrm{sec} = 5000\ \textrm{joules}
Gasoline Usage in the United States
Imagine that we did not have the US Energy Information Administration compiling statistics of gasoline usage. If we needed to make an estimate of gasoline usage to support a decision about the speed limit of fuel consumption how would we make it?