Heat Capacity
Some materials require more energy to be added or removed for the same temperature change as other materials. This property is call heat capacity.
Note that large objects (an ocean being warmed by climate change) can store lots of energy for a small change in temperature. Also note that for equal masses, some materials require less energy for a change in temperature. To understand this we use the terms intensive and extensive.
Intensive vs Extensive Properties
- Intensive or bulk property
- Does not depend on the amount of material
- Example: temperature, density
- Extensive property
- Depends on the amount of material
- Example: mass, volume
Heat Capacity
- When heat moves from one material to another one temperature rises the other falls
- The temperature rise occurs as heat increases the motion of atoms in the material
- The rate of change in temperature with added energy is dictated by the heat capacity
- Ratio of heat added to temperature change
Heat Capacity
- Extensive property (of the material and the amount)
- Measured in Joules per degree Kelvin
Specific Heat Capacity
- Intensive property (of the material only)
- Measured in Joules per mass per degree Kelvin
Table of Specific Heat Values
| Material | Heat Capacity (Joule/gram/kelvin) |
|---|---|
| Air | 1 |
| Water | 4.1813 |
| Iron | 0.45 |
| Aluminum | 0.897 |
| Brick | 0.840 |
| Wood | 1.2 - 2.9 |
Partial list from https://en.wikipedia.org/wiki/Heat_capacity
Heat capacity
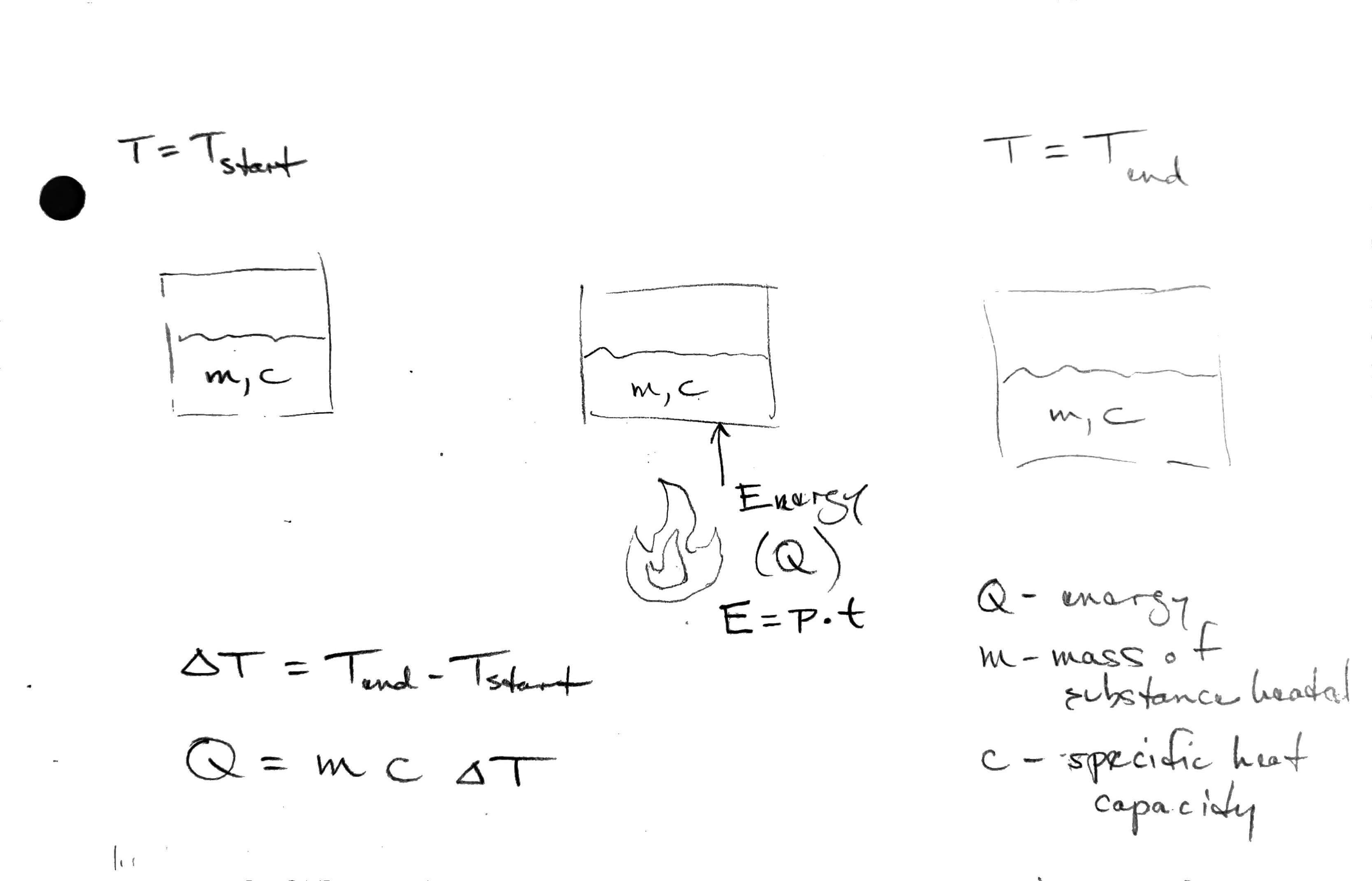
We have an equation that tells us how the energy absorbed by a material is related to the temperature rise.
Q = mc\Delta T
- Q is energy transferred to or from substance
- m is the mass
- c is the specific heat capacity
- \Delta T is the temperature change
Units
| Quantity | Metric Units | Imperial Units |
|---|---|---|
| Energy | Joules | BTU |
| Mass | grams, kg | pounds |
| Temperature | Celsius, Kelvin | Fahrenheit |
| Specific Heat Capacity | Joule / gram / Kelvin | BTU / lb / F |
Useful Conversion Factors
- 1055 Joules = 1 British Thermal Unit (BTU)
- 2.2 pounds (lb) = 1 kilogram (kg)
- 9 Fahrenheit = 5 Celsius
The heat capacity for water is
- 4.2 Joules per gram per Kelvin
- 1.0 BTU per pound per Fahrenheit
Energy required for tea
How much energy does it take to make a cup of tea? Assume the water starts at 20C and is raised to 95C for a delta T of 75C. Further assume that there is 200 ml of water (200 grams).
Q = mc\Delta T
Q = 200 grams \cdot 4.18 Joule/gram/kelvin \cdot 75K
Q = 63 kJ
Graphical Interpretation
In this graph the slope is equal to 1/mc.
Relation to Buildings
- Thermal mass is added to a building to store heat
- Cool or warm air is passed through a building to deliver or remove heat.
Relation to Weather and Climate
- The heat capacity of water affects the climate in some areas
Rate of increase
If we have a source of constant thermal power and assume that all that energy is going into an object, we can calculate the rate of temperature rise of that object.
Q = mc\Delta T
We divide both sides by time.
\frac{Q}{t} = \frac{mc\Delta T}{t}
Remember that Q is an energy. That means Q over t is a power.
P = \frac{mc\Delta T}{t}
If we rearrange this equation algebraically, we see that
\frac{P}{mc} = \frac{\Delta T}{t}
This means that the power divided by the heat capacity gives you an estimate of the rate of temperature increase in an object.
Recall that the power to energy formula is:
E = P \cdot t
In metric units, energy is in Joules, power is in Watts, and time is in seconds.
In imperial units, energy is in British Thermal Units (BTU), power is in British Thermal Units per hours (often called BTU), and time is in hours.