Thermodynamics and Heat Engines
- Heat engines and refrigerators are important in energy use
- A heat engine extracts energy for movement from heat as it flows from hot to cold
- A refrigerator inputs energy to move heat from cold to hot
- The efficiency of these is dictated by thermodynamics
Fundamental Concepts
- Temperature
- Fahrenheit, Celsius, Kelvin Scale
- First Law of Thermodynamics
- Second Law of Thermodynamics
Applied Concepts
- Carnot cycle heat engine
- Carnot refrigerator
- Carnot efficiency
- Coefficient of performance for refrigeration
- Coefficient of performance for heating
Temperature
- Measure of the internal energy in a system or material
- This energy is the motion, vibration, or rotation of atoms and molecules
Zeroth Law of Thermodynamics
- If two systems are each in thermal equilibrium with a third system, they are also in thermal equilibrium with each other.
- Real world example: Coffee gets cold, ice cream melts
First Law of Thermodynamics
- Energy is conserved
- Energy cannot be created or destroyed
- “You can’t get something for nothing”
First Law Efficiency
- Most commonly used measure of efficiency
- Useful energy out divided by total energy in
Second Law of Thermodynamics
- The amount of entropy (disorder) in a closed system always increases
- Heat flows spontaneously from hot to cold
- “You can’t break even”
Second Law Thermodynamic limit to heat engine
- Carnot derived the upper limit of efficiency for a heat engine \eta = 1 - \frac{T_C}{T_H} \eta = \frac{T_H - T_C}{T_H}
- This law dictates the maximum possible efficiency for power plants
- Some of the heat must be released into the environment
Second Law Efficiency
- Compares the efficiency of a system to the maximum possible efficiency given by the temperatures and the second law of thermodynamics
Exploring the Carnot Equation
What is the efficiency of a carnot heat engine when
- T_C is near zero?
- T_H is very high?
- T_C and T_H are close to each other?
Heat Engine Definition
- Heat engines convert thermal energy to mechanical kinetic energy
Heat Engine Examples
- Coal power plant turbines
- Internal combustion engines
Heat Engines
- The heat engine is a mathematical model
- Takes the heat (flow) between two thermal reservoirs and converts some of that heat to work
- Heat can come from combustion or natural sources of heat
- This conversion can never be 100 percent efficient because of the second law
Heat Engines
- A heat engine is more efficient when it uses a wider temperature range between the hot and cold sides
Heat Engine
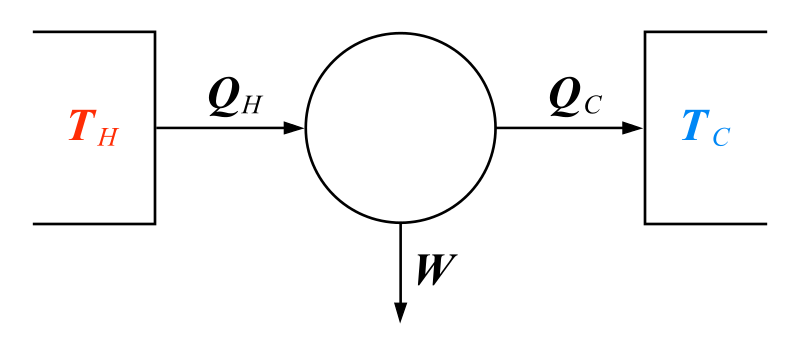
Quality
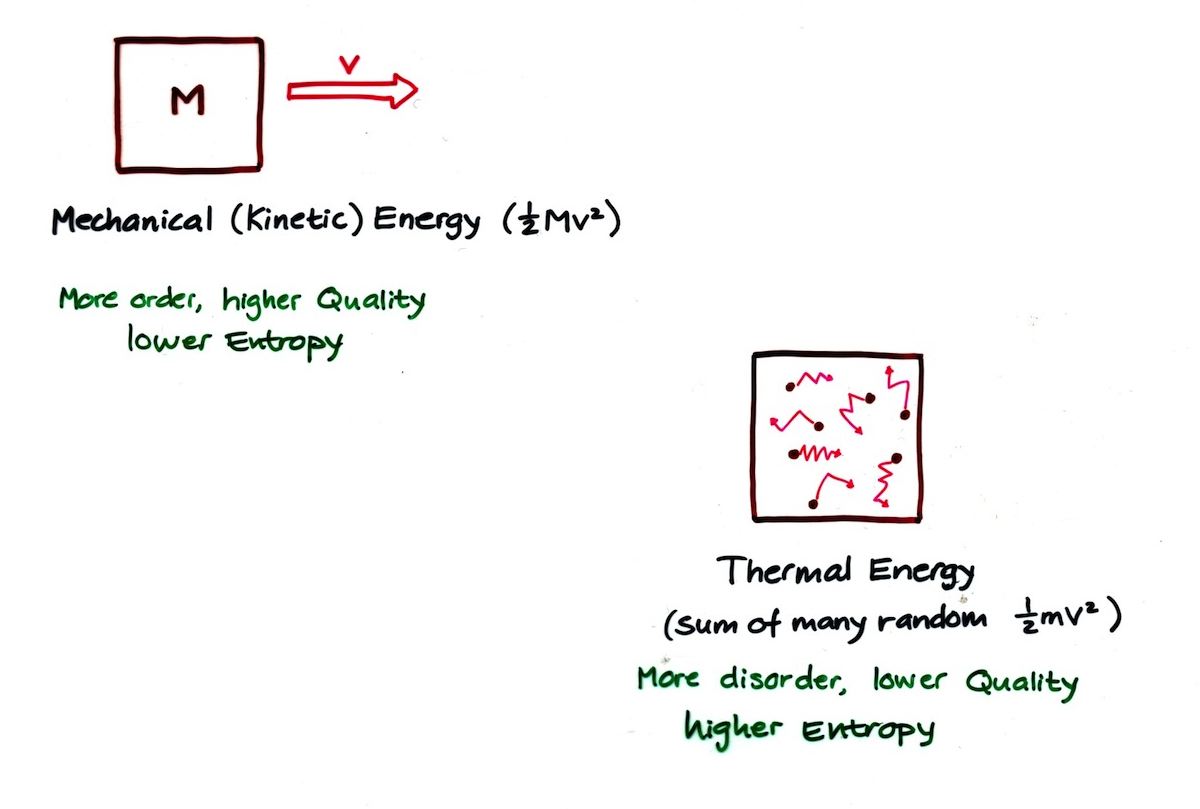
Quality
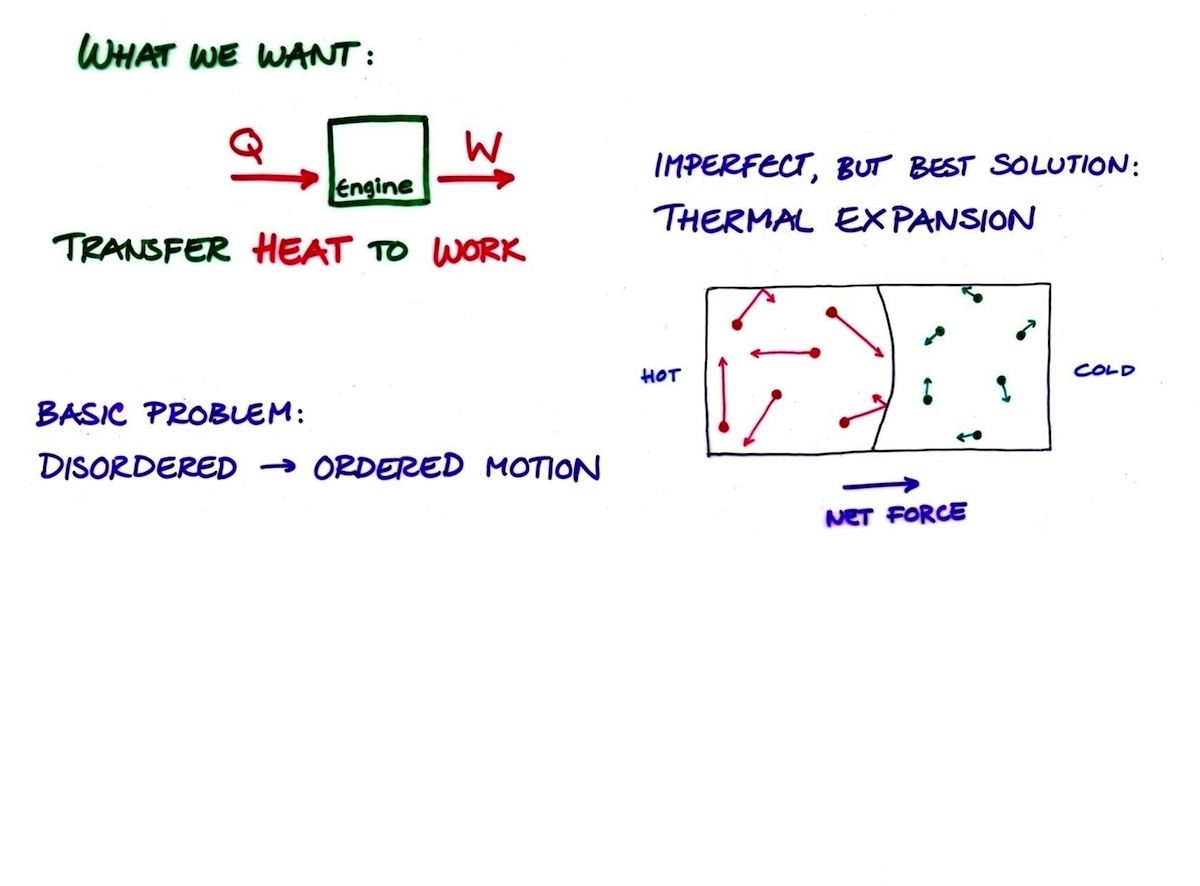
Carnot Heat Engine
- The most efficient heat engine possible uses a Carnot cycle
- Heat is used to expand a gas and do work and heat is removed during the compression of the gas.
- There are many other cycles used in steam generators and internal combustion engines
Power plant
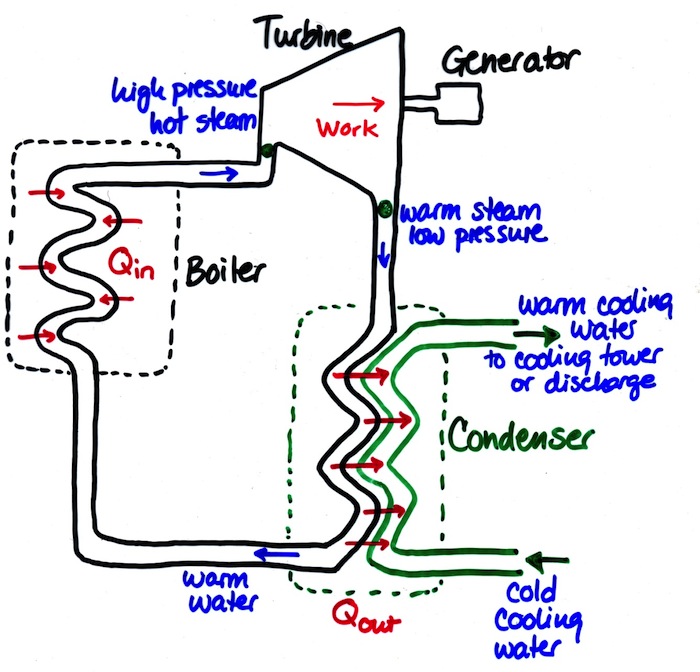
Power plant
